Entrepreneurial Support Program(Explore Course)

- Principal Investigator
-
KEIO University
Norihisa Miki
- Adopted Theme
Implantable Artificial Kidney for Next-Generation Dialysis: From Practical Realization to Global Outreach
- Subject of Research
- Implantable Artificial Kidney for Next-Generation Dialysis: From Practical Realization to Global Outreach
- GTIE VC Collective
KEIO INNOVATION INITIATIVE
- Overview
-
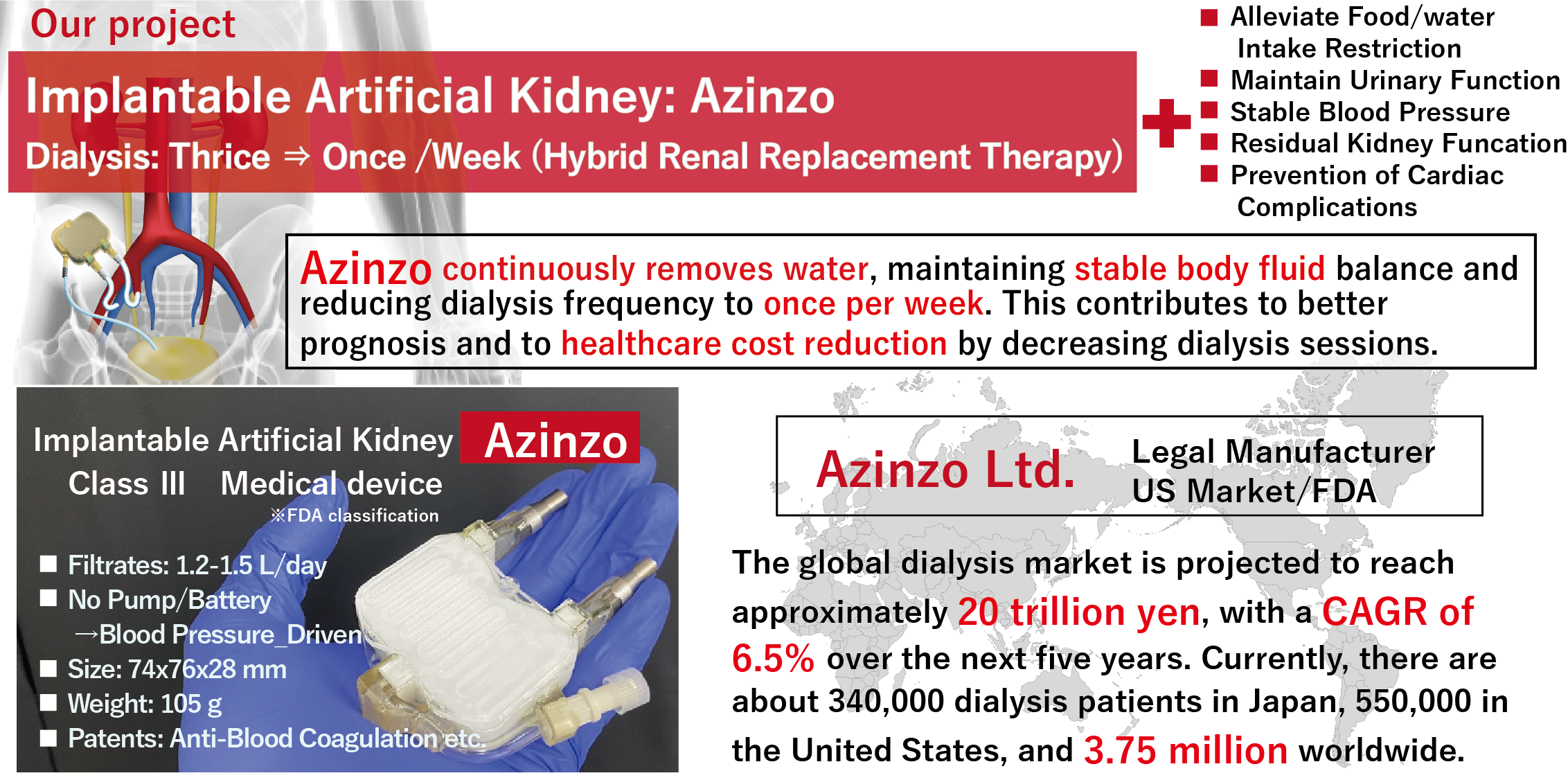
We are advancing the research and development of Azinzo, an implantable artificial kidney, which enables hybrid renal replacement therapy. The new generation therapy using Azinzo is expected to revolutionize the quality of life for end-stage renal disease patients by reducing dialysis frequency to as little as once per week, while providing therapeutic benefits such as stabilized blood pressure and preserved bladder function, alongside significant reductions in healthcare costs.
This project encompasses preclinical evaluation of Azinzo’s safety and efficacy in goat models, establishment of a QMS framework with the U.S. market in mind, and preparations for regulatory approval. Building on over 15 years of research and development, we leverage our patents and proprietary manufacturing know-how to design and produce Azinzo—an unprecedented device with no comparable alternatives—and challenge the enormous global market, projected to reach 20 trillion yen in 2030s.
- Business Models(when applying)
Azinzo Inc. designs, manufactures, and markets the implantable artificial kidney based on proprietary patents and accumulated know-how. High-performance medical-grade materials are sourced from external suppliers, while core technologies are retained in-house to ensure differentiation. By generating robust clinical data and engaging key opinion leaders (KOLs), Azinzo will drive early adoption and gradually expand distribution channels. With regulatory approval centered on the U.S. market, the company will enter the rapidly growing dialysis and renal replacement therapy sector, contributing to both healthcare cost reduction and dramatic improvements in patient quality of life.
- Activity Planning(when applying)
This project pursues the practical application of the implantable artificial kidney Azinzo, advancing both business and research development in parallel. On the business side, we will establish a system that enables prompt QMS operation after incorporation as a Legal Manufacturer, and achieve consensus with the FDA on non-clinical studies through a Pre-submission. On the R&D side, we will perform staged long-term implantation studies in goats—1 week, 1 month, 3 months, and 6 months—developing protocols that address acute postoperative inflammatory responses and conducting cadaver studies to finalize device specifications. Each year, we will also engage with KOLs through international conferences and refine our business plan in discussion with investors. Through these steps, we aim to secure a robust QMS framework, qualified personnel, and a precise development roadmap, thereby building the foundation for rapid business acceleration after incorporation and positioning Azinzo for global market entry.