Entrepreneurial Support Program(Oversea market pioneer practice Course)

- Principal Investigator
-
Institute of Science Tokyo
Takuya Nagano
- Adopted Theme
Development of stereotactic body radiation therapy using a novel fixation device for early-stage tongue cancer
- Subject of Research
- Development of stereotactic body radiation therapy using a novel fixation device for early-stage tongue cancer
- Overview
-
Stereotactic Body Radiation Therapy (SBRT) has been applied to early-stage lung cancer, demonstrating high local control and a low incidence of adverse events. This therapy involves irradiating small tumors, less than 5 cm in size, from multiple directions, focusing high doses of radiation exclusively on the tumor with an accuracy margin of less than 2 mm for head and neck tumors. Although tongue cancer is also a "moving tumor," it could potentially be treated with SBRT if the tongue can be immobilized and the appropriate irradiation area accurately identified.
Therefore, we conceived the idea that SBRT for tongue cancer might be possible using a tongue suction fixation spacer and oral markers. If the efficacy and safety of this treatment can be confirmed, it could resolve the issue of reduced quality of life (QOL) post-treatment and allow for minimally invasive treatment to be widely adopted in general hospitals nationwide. Our clinical research has shown that the tongue can be immobilized with an accuracy of 0.6 mm. Moving forward, we aim to conduct clinical trials in the United States, obtain FDA approval, and bring this treatment to market.
- Business Models(when applying)
Our business model involves supporting SBRT for American patients with tongue cancer by providing custom-made spacers and oral markers. Based on oral scanned 3D data, MRI, and CT images, we design optimal spacers and print them using a 3D printer at our American factories. These spacers and oral markers are then shipped to the patients, along with information on the expected irradiation planß and potential side effects. We charge patients $5,000 for these services.
- Activity Planning(when applying)
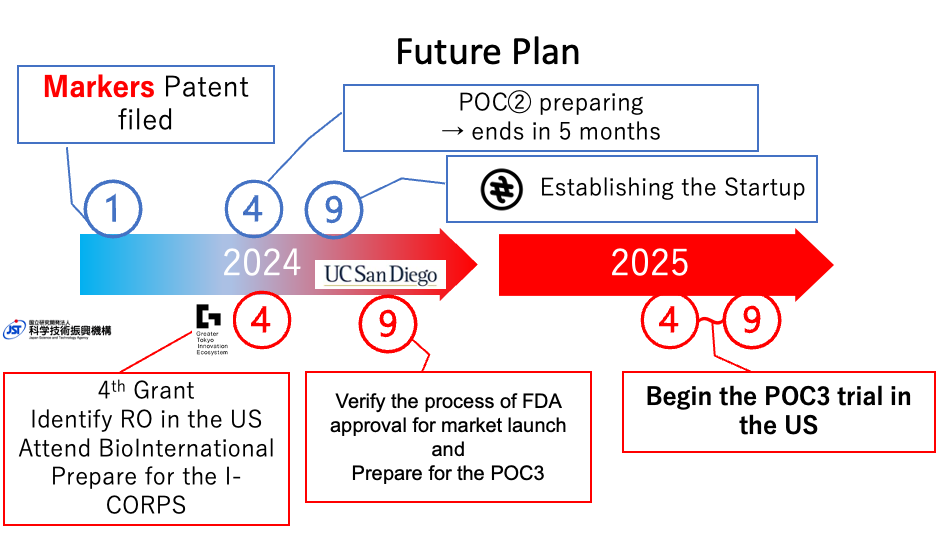
1. At Bio International, we will establish a network with hospitals renowned for head and neck cancer radiation therapy and search for a suitable CEO candidate. If there are promising candidates, we will directly approach them and explain our business plan.
2. We will prepare for the clinical research (POC②) scheduled for August for tongue cancer. This involves procuring necessary equipment and materials, creating the research protocol, and submitting applications to the ethics committee to ensure smooth research implementation.
3. Based on the results of POC②, we will conduct the first-in-human treatment for a tongue cancer patient either in the United States or Japan. Using these results, we aim to secure funding from venture capitalists. To achieve this, we will create a business plan, prepare presentation materials, and network with investors.
Through these activities, we aim to make steady progress towards the practical application of innovative SBRT for tongue cancer, contributing to improved patient quality of life and the spread of minimally invasive treatments.