Entrepreneurial Support Program(Explore Course)

- Principal Investigator
-
Tokai University
Takeharu Hayashi
- Adopted Theme
Toward Delivering a Novel Therapy for Cardiomyopathy, a Designated Intractable Disease
- Subject of Research
- Toward Delivering a Novel Therapy for Cardiomyopathy, a Designated Intractable Disease
- GTIE VC Collective
Mitsubishi UFJ Capital Co., Ltd.
- Overview
-
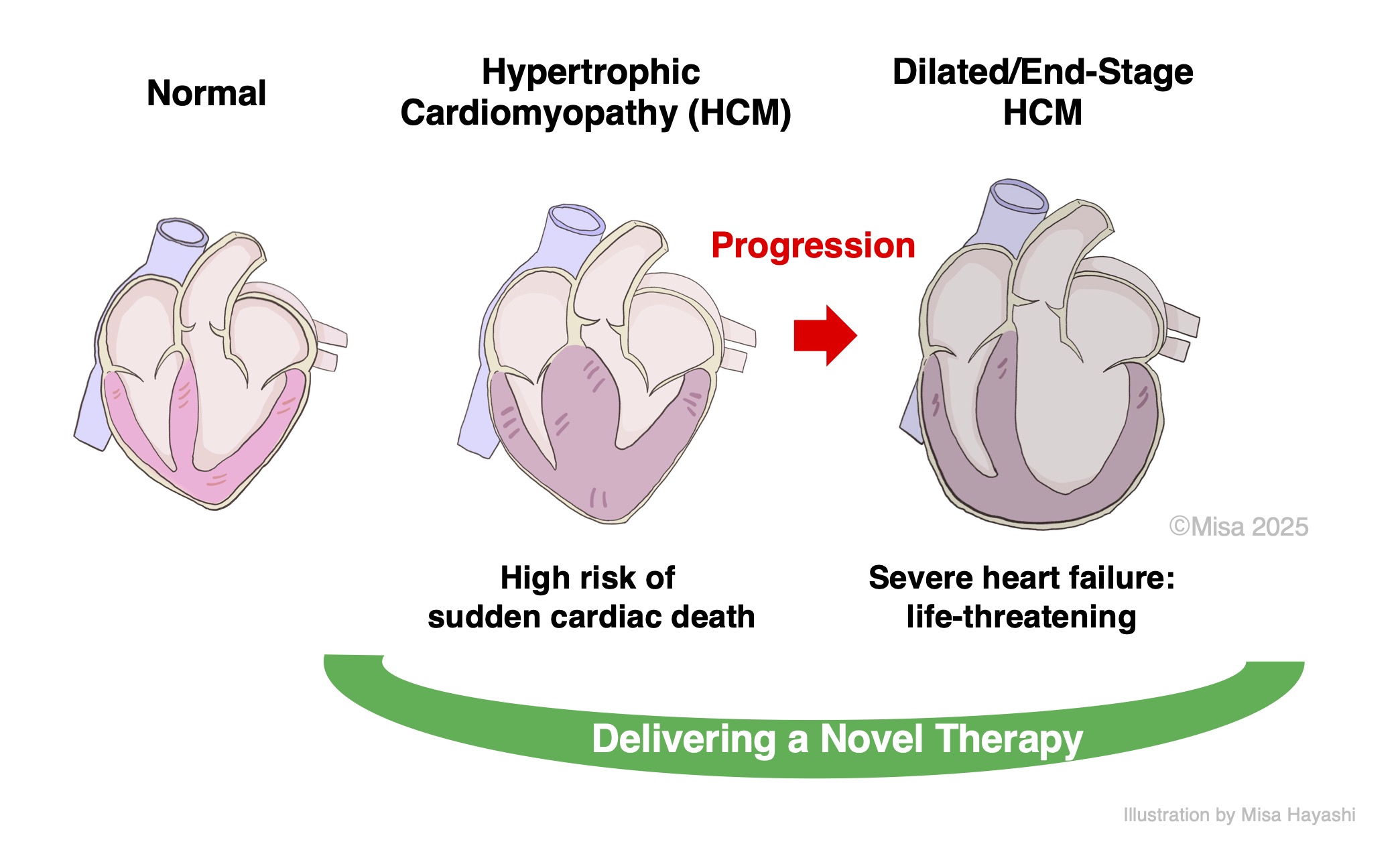
Hypertrophic cardiomyopathy (HCM), a major cause of refractory heart failure and the leading cause of sudden cardiac death in the young, is designated as an intractable disease in Japan. A subset of patients progresses to a dilated phase, end-stage phenotype with severe heart failure and is sometimes diagnosed only at that stage. However, current therapies are basically symptomatic and do not halt disease progression. We have discovered a novel therapeutic protein with anti-hypertrophic activity and aim to develop and provide it as a first-in-class treatment for cardiomyopathy. We will optimize the formulation and evaluate efficacy across multiple disease models, along with pharmacokinetics, safety, and stability, in a staged program, and advance to clinical trials. By strengthening international patents and collaborating with global pharmaceutical companies, we will accelerate worldwide development. Our goal is to deliver a therapy that improves prognosis and quality of life and reduces the healthcare burden as swiftly and responsively as possible.
- Business Models(when applying)
We will establish a drug-discovery startup and advance development centered on a novel protein-based medicine for cardiomyopathy. We will verify efficacy and safety and build a scalable manufacturing scheme. Funding will be secured through government grants and partner venture capital funds, and we will leverage trusted external partners (CDMOs/CROs) for manufacturing and testing. After progressing the asset in-house from preclinical through Phase II to maximize value, we plan to commercialize via out-licensing or M&A with global pharmaceutical companies, generating upfront payments, development milestones, and sales-based royalties.
- Activity Planning(when applying)
We will refine a protein whose novel effects we have identified into an optimal, clinically viable formulation. In addition to evaluating efficacy across multiple cardiomyopathy models, we will systematically assess safety, pharmacokinetics, and stability. In parallel, we will establish quality control and scalable manufacturing, structure the development pathway, and engage early with regulatory authorities to translate non-clinical proof of concept into a clinical program. We will further define the target patient population, primary clinical endpoints, and dosing regimen; enter the national phase of our international patent applications and conduct competitive landscape analysis; prepare for partnerships and out-licensing with global pharmaceutical companies; and, in a coordinated manner, build the financing plan and organizational capabilities across management, regulatory, and CMC, while completing readiness for clinical-trial-material manufacturing.