Entrepreneurial Support Program(Explore Course)

- Principal Investigator
-
Institute of Science Tokyo
Kohji Seio
- Adopted Theme
Development of therapeutic drugs through control of extracellular matrix remodeling using antisense nucleic acids
- Subject of Research
- Development of therapeutic drugs through control of extracellular matrix remodeling using antisense nucleic acids
- GTIE VC Collective
Beyond Next Ventures Inc.
- Overview
-
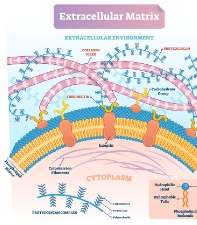
The extracellular matrix (ECM) is an interstitial substance that exists around cells and is composed of many components. It is known that ECM remodeling plays an important role in various diseases as well as in the maintenance of normal tissues. This project aims to develop a nucleic acid medicine targeting an ECM regulatory molecule (tentative name: Factor-X), which is a joint seed project between Tokyo Institute of Technology and Showa University, as a therapeutic drug for multiple diseases involving ECM remodeling, and to commercialize the medicine to improve the quality of life of patients suffering from diseases for which there is currently no effective treatment. The project will be accomplished through close collaboration among experts in the three fields of medicine, engineering, and business.
- Business Models(when applying)
We aim to establish a bio-venture company with a drug discovery pipeline for multiple diseases up to around phase 2 clinical trials, with the goal of developing drugs by regulating the extracellular matrix targeting Factor-X (tentative name).
- Activity Planning(when applying)
- Confirmation of Factor-X regulatory effects of the lead compound, a nucleic acid medicine (patented technology), in cells related to each disease
- Confirmation of therapeutic effects of lead compounds on model cells and animals for each disease
- Improvement of efficacy against each disease and reduction of side effects by chemical modification of lead compounds
- Optimization of chemical structure by feedback of experimental results
- Optimization of drug efficacy and safety by optimizing the combination of administration and delivery methods
- Evaluation of development plans for non-clinical and clinical studies
- Examination of commercialization plan for fundraising and international development